No products in the cart.
Montelukast EP Impurity H

Product Description
CAT No.
ALN-M031028
CAS No.
851755-56-1
Mol. F.
C34H32ClNO4S
Mol. Wt.
586.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
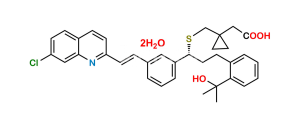
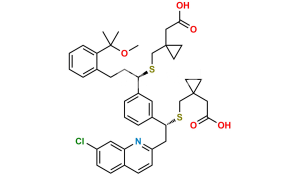
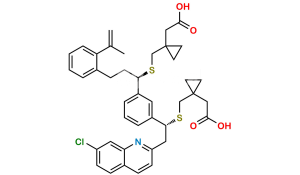
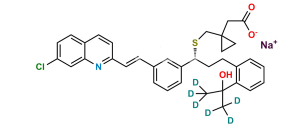
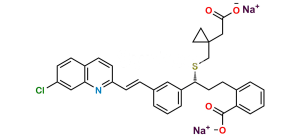
Chemical Name : [1-[[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(methoxycarbonyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl]acetic acid (as per EP)
Smiles : O=C(C1=CC=CC=C1CC[C@@H](SCC2(CC2)CC(O)=O)C3=CC=CC(/C=C/C4=NC5=CC(Cl)=CC=C5C=C4)=C3)OC
Inchi : InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10-/t32-/m1/s1
Synonym : Montelukast Methoxycarbonyl Analog
Technical Data
Reference
Method development and validation for the simultaneous estimation of montelukast and acebrophylline in bulk and tablet dosage forms by RP-HPLC
By Ankitha, M.; Ashitha, S.; Sri, Ragasudha A.; Latha, Madhavi P. V.; Devi, Uma P.nFrom Journal of Global Trends in Pharmaceutical Sciences (2021), 12(1), 9173-9179
Montelukast microsuspension with hypromellose for improved stability and oral absorption
By Lee, Ha Ryeong; Park, Hyun Jin; Park, Jun Soo; Park, Dong Woo; Ho, Myoung Jin; Kim, Dong Yoon; Lee, Hyo Chun; Kim, Eun Jeong; Song, Woo Heon; Park, Jun Sang; et al – From International Journal of Biological Macromolecules (2021), 183, 1732-1742
Analytical method development and validation for simultaneous estimation of Bilastine and Montelukast sodium by UV spectrophotometry
By Raj, R. Mohan; Sankar, A. S. K.; Vetrichelvan, T. – From World Journal of Pharmacy and Pharmaceutical Sciences (2021), 10(1), 680-687
RFQ