No products in the cart.
Nadolol Citrate Ester

Product Description
CAT No.
ALN-N002016
CAS No.
NA
Mol. F.
C23H33NO10
Mol. Wt.
483.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
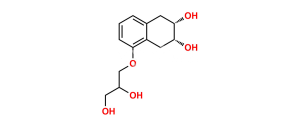
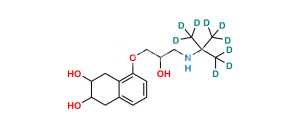
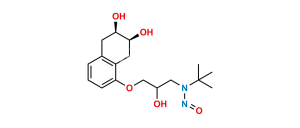
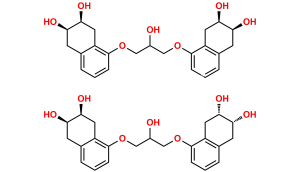
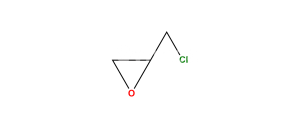
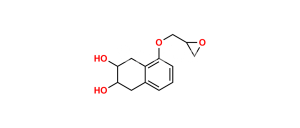
Chemical Name : 2-(((5-(3-(Tert-butylamino)-2-hydroxypropoxy)-1,2,3,4-tetrahydro-2-hydroxynaphthalen-3-yloxy)carbonyl)-methyl)-2-hydroxysuccinic acid
Smiles : O=C(O)C(O)(CC(OC1C(O)CC2=C(C(OCC(O)CNC(C)(C)C)=CC=C2)C1)=O)CC(O)=O
Inchi : InChI=1/C20H31N3O4S2/c1-16-15-21-9-6-19(16)23-10-7-18-14-17(4-5-20(18)23)8-12-29(26,27)22(2)11-13-28(3,24)25/h4-5,7,10,14,16,19,21H,6,8-9,11-13,15H2,1-3H3
Technical Data
Reference
Nadolol: high-pressure liquid chromatographic methods for assay, racemate composition and related compounds
pauline m. lacroix,t norman m. curran and edward g. loveringnJournal of Pharmaceutical & Biomedical Analysis Vol. 10, Nos 10-12, pp. 917-924, 1992
The nonmetabolized u03b2u2011blocker nadolol is a substrate of oct1, oct2, mate1, mate2-k, and pu2011glycoprotein, but not of oatp1b1 and oatp1b3
Shingen Misaka, Jana Knop, Katrin Singer, Eva Hoier, Markus Keiser, Fabian Mu?ller, Hartmut Glaeser, Jo? rg Ko? nig, and Martin F. Fromm – Mol. Pharmaceutics 2016, 13, 2, 512–519
Separation of nadolol racemates by high pH reversed-phase preparative chromatography
Rami S. Arafaha,b , António E. Ribeiroa,b , Alírio E. Rodriguesc , Luís S. Pais – Separation and Purification Technology Volume 233, 15 February 2020, 116018
RFQ