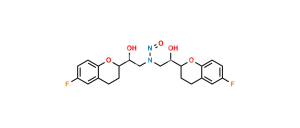
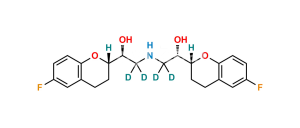
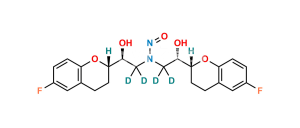
Nebivolol Impurity 34 (RSRR+RRRR)

Product Description
CAT No.
ALN-N020046
CAS No.
NA
Mol. F.
C44H50F4N2O8
Mol. Wt.
810.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
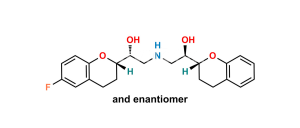
Chemical Name : H-NBH isomer (Mixture of RSRR +RRRR Isomers)
Smiles : O[C@H]([C@@H]1OC2=CC=C(F)C=C2CC1)CNC[C@@H](O)[C@H]3CCC(C=C(F)C=C4)=C4O3.O[C@@H]([C@@H]5OC6=CC=C(F)C=C6CC5)CNC[C@@H](O)[C@H]7CCC(C=C(F)C=C8)=C8O7
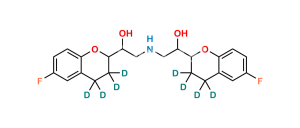
Inchi : InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)/i2D3,6D2
Technical Data
Reference
Development and validation of a stability-indicating reverse phase ultra performance liquid chromatographic method for the estimation of nebivolol impurities in active pharmaceutical ingredients and pharmaceutical formulation
Veera Raghava Raju Thummala, Mohana Krishna LankanSe Pu. 2015 Oct;33(10):1051-8.
Stability-Indicating RP-UPLC Method Development and Validation for the Process Related Impurities of Nebivolol and Structural Characterization of Its Forced Degradation Products by LC-MS/MS
Prasad Kancherla1
Liquid chromatographic impurity profiling of Nebivolol Hydrochloride from bulk drug
, Pallavi Alegete1 , Srinivas Keesari2 , Bhavyasri Khagga1 , Sridhar Siddiraju3 , Mukkanti Khagga1 and Parthasarathi Das – British Journal of Pharmaceutical Research 14(6): 1-13, 2016
RFQ