No products in the cart.
Nifedipine Impurity 6

Product Description
CAT No.
ALN-N003014
CAS No.
NA
Mol. F.
C25H35N3O7S
Mol. Wt.
521.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
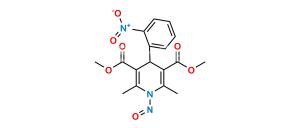
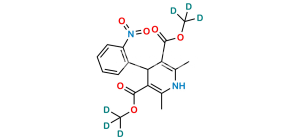
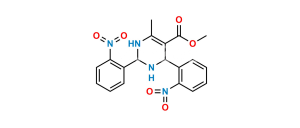
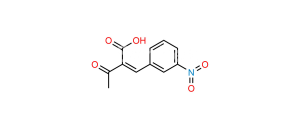
Chemical Name : (1S, 2R)-Benzyl-3-[n-butyl (4-nitrobenzenesulfonyl)-amino]-2-hydroxypropyl carbamic acid tert-butyl ester
Smiles : O=C(OC(C)(C)C)N[C@@H](CC1=CC=CC=C1)[C@H](O)CN(CCCC)S(=O)(C2=CC=C([N+]([O-])=O)C=C2)=O
Inchi : InChI=1S/C24H33N3O7S/c1-5-15-26(35(32,33)20-13-11-19(12-14-20)27(30)31)17-22(28)21(16-18-9-7-6-8-10-18)25-23(29)34-24(2,3)4/h6-14,21-22,28H,5,15-17H2,1-4H3,(H,25,29)/t21-,22+/m0/s1
Technical Data
Reference
Rapid characterization of impurities in the bulk drug of nifedipine by high performance liquid chromatography-quadrupole time of fight mass spectrometry
Peixi Zhu 1, Lixi Ding, Jiajia He, Guogang ZhengnSe Pu. 2012 Oct;30(10):1026-30.
Development and validation of rp-hplc method for the estimation of process related impurity from nifedipine
Dr.Manisha. S. Kedar1
Validation of an HPLC Method for Nifedipine and its Related Substances in Raw Materials
, Dr.Manoj. P. Shirbhate2 , Manjusha. S. Sanap3 , Dr.Rani. S. Kankate4 , Prerana. B. Jadhav5 and Bhagyashri. J. Warude – European Journal of Molecular & Clinical Medicine Volume 7, Issue 8, 2020; 5593-5601
RFQ