No products in the cart.
Norethindrone EP Impurity B

Product Description
CAT No.
ALN-N012002
CAS No.
734-32-7
Mol. F.
C18H24O2
Mol. Wt.
272.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
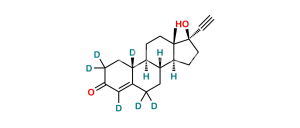
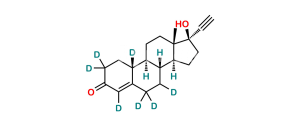
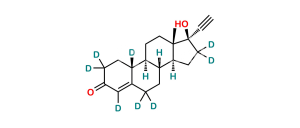
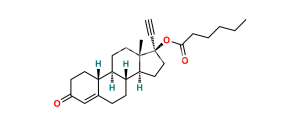
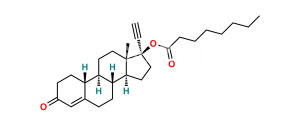
Chemical Name : Estr-4-ene-3,17-dione (as per EP);
Estr-4-ene-3,17-dione (as per USP)
Smiles : O=C(C=C1CC[C@@]2([H])[C@]3([H])CC4)CC[C@]1([H])[C@@]2([H])CC[C@]3(C)C4=O
Inchi : InChI=1S/2C41H46ClNO5S2/c2*1-39(2,48)33-9-4-3-6-27(33)12-15-35(49-25-40(16-17-40)23-37(44)45)29-7-5-8-30(20-29)36(50-26-41(18-19-41)24-38(46)47)22-32-14-11-28-10-13-31(42)21-34(28)43-32/h2*3-11,13-14,20-21,35-36,48H,12,15-19,22-26H2,1-2H3,(H,44,45)(H,46,47)/t35-,36+;35-,36-/m11/s1
Synonym : norandrostenedione (EP) ; Norethindrone USP Related Compound B
Technical Data
Reference
Method development and validation of norethindrone acetate assay and its related impurities in API and pharmaceutical formulation with ortogonal detector technques
By Satish, J.; Radhakrishnanand, P.; Babu, K. SurendranFrom International Journal of Pharmacy and Pharmaceutical Sciences (2017), 9(12), 110-118
Reversed-phase high-performance liquid chromatographic determination of Norethindrone acetate and ethinyl estradiol in pharmaceutical formulation
By Koneru, Ajitha; Kimbahune, Ritu; Mubeen, G.; Lalitha, N.; Chakraborty, Krishnasis – From Indo American Journal of Pharmaceutical Research (2015), 5(6), 2442-2449
Development and validation of method for the determination of related substances of norethindrone in norethindrone tablets and degradation studies
By Murali Krishna, P.; Thirupathi Rao, B.; Kishore Kumar, R.; Venkateswarlu, P. – From International Journal of ChemTech Research (2011), 3(1), 143-148
RFQ