No products in the cart.
Olmesartan Impurity 12

Product Description
CAT No.
ALN-O006049
CAS No.
NA
Mol. F.
C50H48N6O6
Mol. Wt.
829
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
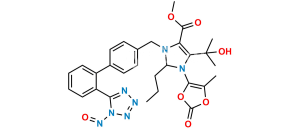
Chemical Name : (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-butyl-4-(2-hydroxybutan-2-yl)-1-((2′-(1-trityl-1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl)methyl)-1H-imidazole-5-carboxylate
Smiles : CCCCC1=NC(C(CC)(O)C)=C(C(OCC2=C(C)OC(O2)=O)=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NN=NN5C(C6=CC=CC=C6)(C7=CC=CC=C7)C8=CC=CC=C8
Inchi : InChI=1S/C49H46N6O6/c1-5-6-26-42-50-44(48(3,4)58)43(46(56)59-32-41-33(2)60-47(57)61-41)54(42)31-34-27-29-35(30-28-34)39-24-16-17-25-40(39)45-51-52-53-55(45)49(36-18-10-7-11-19-36,37-20-12-8-13-21-37)38-22-14-9-15-23-38/h7-25,27-30,58H,5-6,26,31-32H2,1-4H3
Technical Data
Reference
Development and Validation of a Stability-Indicating Liquid Chromatographic Method for Estimating Olmesartan Medoxomil Using Quality by Design
Sarwar Beg, Gajanand Sharma, O.P. Katare, Shikha Lohan, Bhupinder SinghnJournal of Chromatographic Science, Volume 53, Issue 7, August 2015, Pages 1048u20131059
Stability Indicating RP-HPLC Method Development and Validation for Simultaneous Quantification of 15 Organic Impurities of Olmesartan Medoxomil, Amlodipine and Hydrochlorothiazide in Combined Dosage Form
Pritesh R. Desai, Priti J. Mehta & Avani B. Chokshi – Chromatographia volume 82, pages819–833(2019)
RFQ