No products in the cart.
Omeprazole Impurity 16

Product Description
CAT No.
ALN-O004053
CAS No.
106135-28-8
Mol. F.
C8H8N2O4S
Mol. Wt.
228.2
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
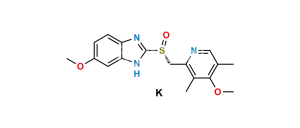
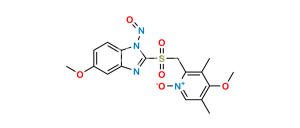
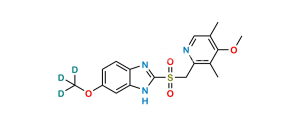
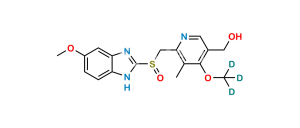
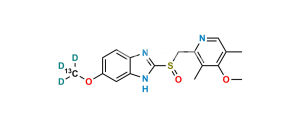
Chemical Name : 5-Methoxy-1H-benzo[d]imidazole-2-sulfonic acid
Smiles : O=[S](O)(C1=NC(C=C(OC)C=C2)=C2N1)=O
Inchi : InChI=1S/C22H42O3/c1-3-5-7-9-10-11-12-13-14-16-19(23)18-21-20(22(24)25-21)17-15-8-6-4-2/h19-21,23H,3-18H2,1-2H3/t19-,20+,21+/m0/s1
Technical Data
Reference
Elemental impurities analysis in name-brand and generic omeprazole drug samples
Fernanda C. Pinheiro, Ariane I.Barros, Joaquim A.Nu00f3breganHeliyon Volume 6, Issue 2, February 2020, e03359
Development of a validated RP-HPLC method for separation and determination of process-related impurities of omeprazole in bulk drugs
cristina iuga, marius boji??, sorin e. leucu?a – farmacia, 2009, vol. 57, 5
Low Level Quantification of Potential Genotoxic Impurity in Omeprazole Drug Substance by UPLC
Vinay Kumar Patcha , Susheela Bhai Gajbhiye , Vundavilli Jagadeesh Kumar , Ray UK ,Pavan Kumar KSR , Sreenivas N – Der Pharma Chemica, 2017, 9(15):72-76
RFQ