No products in the cart.
Ondensatron Homo Impurity
Product Description
CAT No.
ALN-O002012
CAS No.
NA
Mol. F.
C19H19N3O
Mol. Wt.
305.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
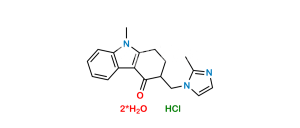
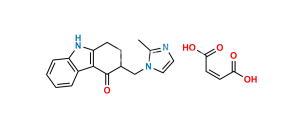
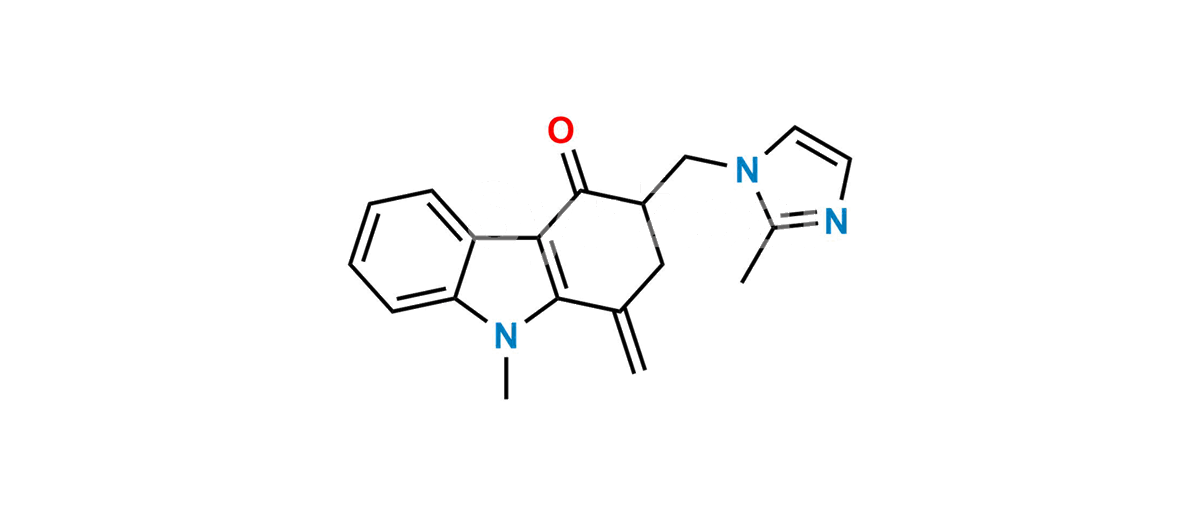
Chemical Name : 9-Methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-1-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one
Smiles : O=C1C(CN2C(C)=NC=C2)CC(C3=C1C4=CC=CC=C4N3C)=C
Inchi : InChI=1S/C6H18N4O/c7-1-4-10(11,5-2-8)6-3-9/h1-9H2
Technical Data
Reference
Titania-based stationary phase in separation of ondansetron and its related compounds
Vu00e1clavu017diu017ekovsku00fd, RadimKuu010dera, Jiu0159u00edKlimeu0161, Jiu0159u00edDohnalnJournal of Chromatography A Volume 1189, Issues 1u20132, 2 May 2008, Pages 83-91
Degradation of ondansetron: Isolation and characterization impurity D ondansetron as A candidate reference standard impurity in drug
E. Kristiana, Asep Saefumillah, Emil Budianto – AIP Conference Proceedings 2242(1):040054
Identification, isolation and characterization of unknown impurity in Ondansetron Drug product
Hemant Madhusudan Gandhi, Nageswara Rao Gollapalli, Dr. Jaydeep Kumar D. Lilakar – International Journal of Advance Research, Ideas and Innovations in Technology (Volume 4, Issue 2) 1264-1271
RFQ