No products in the cart.
Paroxetine Hemihydrate EP Impurity F

Product Description
CAT No.
ALN-P012014
CAS No.
2105932-71-4
Mol. F.
C39H40F2N2O6
Mol. Wt.
670.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
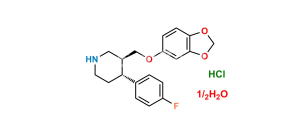
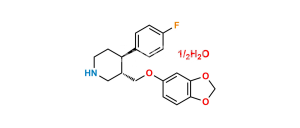
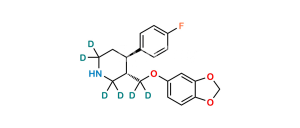
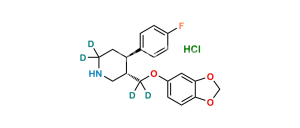
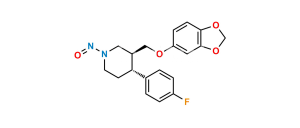
Chemical Name : 3,3′-[methylenebis(1,3-benzodioxole-6,5-diyloxymethylene)]bis[3S,4R)4-(4-fluorophenyl)piperidine]
Smiles : FC1=CC=C([C@@]2([H])[C@@](COC(C(CC3=CC4=C(C=C3OC[C@]5([H])CNCC[C@@]5([H])C6=CC=C(F)C=C6)OCO4)=C7)=CC8=C7OCO8)([H])CNCC2)C=C1
Inchi : InChI=1S/C20H23NO4.ClH/c1-22-16-4-2-14(3-5-16)18-8-9-21-11-15(18)12-23-17-6-7-19-20(10-17)25-13-24-19;/h2-7,10,15,18,21H,8-9,11-13H2,1H3;1H/t15-,18-;/m0./s1
Technical Data
Reference
Development, validation and transfer into a factory environment of a liquid chromatography tandem mass spectrometry assay for the highly neurotoxic impurity FMTP (4-(4-fluorophenyl)-1-methyl-1,2,3,6-tetrahydropyridine) in paroxetine active pharmaceutical ingredient (API)
Phil J.Borman, Marion J.Chatfield, Elizabeth L.Crowley, ChristineEckers, David P.Elder, Scott W.Francey, Alice M.-F.Laures, Jean-ClaudeWolffnJournal of Pharmaceutical and Biomedical Analysis Volume 48, Issue 4, 1 December 2008, Pages 1082-1089
An Investigation into the Dehydration Behavior of Paroxetine HCl Form I Using a Combination of Thermal and Diffraction Methods: The Identification and Characterization of a New Anhydrous Form
M. Fátima Pina, Min Zhao, João F. Pinto, João J. Sousa, Christopher S. Frampton, Victor Diaz, Osama Suleiman, László Fábián, and Duncan Q. M. Craig – Cryst. Growth Des. 2014, 14, 8, 3774–3782
Determination of residual solvents in paroxetine by headspace gas chromatography
abdul rahaman sk, padmavathi sakinala, khaleel n, harekrishna roy – Asian J Pharm Clin Res, Vol 12, Issue 6, 2019, 150-155
RFQ