No products in the cart.
Pazopanib Impurity 21

Product Description
CAT No.
ALN-P013030
CAS No.
1821666-75-4
Mol. F.
C21H21N7O3S
Mol. Wt.
451.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
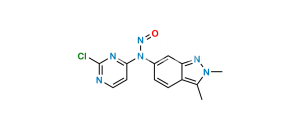
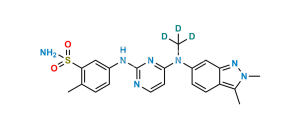
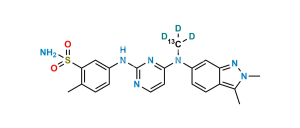
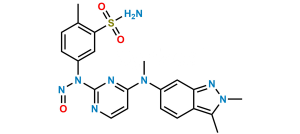
Chemical Name : 5-((4-((3-formyl-2-methyl-2H-indazol- 6-yl) (methyl) amino) pyrimidin-2-yl) amino)-2-methyl benzene sulfonamide
Smiles : O=S(C1=CC(NC2=NC=CC(N(C3=CC4=NN(C)C(C=O)=C4C=C3)C)=N2)=CC=C1C)(N)=O
Inchi : InChI=1S/C21H21N7O2S/c1-13-3-4-14(11-19(13)31(22,29)30)24-21-23-9-7-20(25-21)27(2)15-5-6-16-17(12-15)26-28-10-8-18(16)28/h3-7,9,11-12H,8,10H2,1-2H3,(H2,22,29,30)(H,23,24,25)
Technical Data
Reference
Analytical control of process impurities in Pazopanib hydrochloride by impurity fate mapping
Yan Li 1, David Q Liu, Shawn Yang, Ravinder Sudini, Michael A McGuire, Dharmesh S Bhanushali, Alireza S KordnJ Pharm Biomed Anal. 2010 Aug 1;52(4):493-507.
Determination and characterization of process impurities in pazopanib hydrochloride drug substance
musty sharada, ravichandra babu – Int J Pharm Pharm Sci, Vol 8, Issue 4, 97-102
Characterization of forced degradation products of pazopanib hydrochloride by UHPLC?Q?TOF/MS and in silico toxicity prediction
Prinesh N. Patel\xa0 Pradipbhai D. Kalariya\xa0 Mahesh Sharma\xa0 Prabha Garg\xa0 M. V. N Kumar Talluri\xa0 S. Gananadhamu\xa0 R. Srinivas – Journal of mass spectrometry Volume50, Issue7 July 2015 Pages 918-928
RFQ