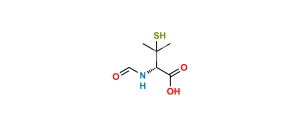
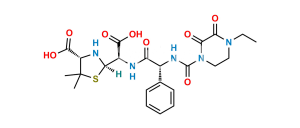
Piperacillin EP Impurity P

Product Description
CAT No.
ALN-P048023
CAS No.
NA
Mol. F.
C31H34N6O8S
Mol. Wt.
650.7
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
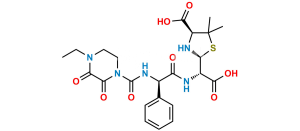
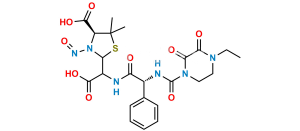
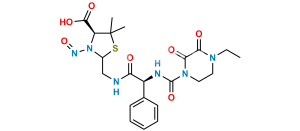
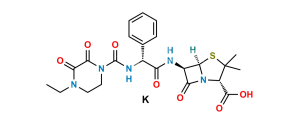
Chemical Name : (2S,5R,6R)-6-[(2R)-2-[(2R)-2-(4-ethyl-2,3-dioxopiperazine-1-carboxamido)-2-phenylacetamido]-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (as per EP)
Smiles : O=C1N(C(N[C@H](C2=CC=CC=C2)C(N[C@@H](C(N[C@]3([H])C(N4[C@]3([H])SC(C)(C)[C@@H]4C(O)=O)=O)=O)C5=CC=CC=C5)=O)=O)CCN(CC)C1=O
Inchi : InChI=1S/C23H29N5O8S/c1-4-27-10-11-28(19(31)18(27)30)22(36)25-13(12-8-6-5-7-9-12)16(29)24-14(20(32)33)17-26-15(21(34)35)23(2,3)37-17/h5-9,13-15,17,26H,4,10-11H2,1-3H3,(H,24,29)(H,25,36)(H,32,33)(H,34,35)/t13-,14-,15+,17+/m1/s1
Technical Data
Reference
Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 2: stability evaluation
By D’cunha, Ronilda; Bach, Thanh; Young, Beth Ann; Li, Peizhi; Nalbant, Demet; Zhang, Jun; Winokur, Patricia; An, GuohuanFrom Antimicrobial Agents and Chemotherapy (2018), 62(9), e00861-18/1-e00861-18/9
Stability indicating RP-HPLC method for the determination of Piperacillin and Tazobactam and their related substances in bulk and pharmaceutical formulation
RFQ