Raltegravir EP Impurity A Hydrobromide salt
Product Description
CAT No.
ALN-R009019
CAS No.
1415836-94-0
Mol. F.
C16H19FN4O3 : HBr
Mol. Wt.
334.4 : 80.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
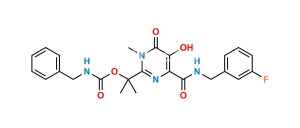
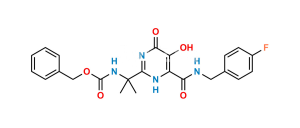
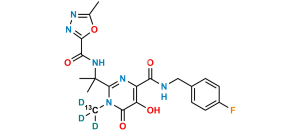
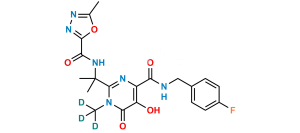
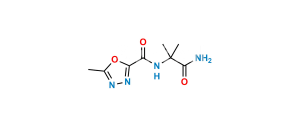
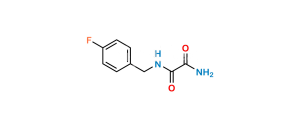
Chemical Name : 2-(2-aminopropan-2-yl)-N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidine-4-carboxamide Hydrobromide salt (as per EP);
2-(2-Aminopropan-2-yl)-N-(4-fluorobenzyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidine-4-carboxamide Hydrobromide salt (as per USP)
Smiles : CN1C(C(C)(N)C)=NC(C(NCC2=CC=C(F)C=C2)=O)=C(O)C1=O.Br
Synonym : Raltegravir amine Impurity
Technical Data
Reference
Development and validation of stability indicating RP-HPLC assay method of raltegravir in tablet dosage forms
By Shirisha, V.; Sairaju, B.; Illendula, Santhosh; Dutt, K. RajeswarnFrom World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(10), 668-692
Stability indicating reverse-phase high performance liquid chromatography method for the determination of raltegravir in bulk and pharmaceutical formulation
By Annapurna, Mukthinuthalapati Mathrusri; Teja, Gunnam Ravi; Hemchand, S.; Babu, R. Ravi Chandra – From International Journal of Green Pharmacy (2018), 12(1Suppl.), S177-S180
Development and validation of RP-HPLC method for determination of raltegravir and its impurities in bulk drug and dosage forms
By Balaji, M.; AppaRao, K. M. Ch.; Ramakrishna, K.; Srinivasarao, V. – From Pharma Science Monitor (2014), 5(3Suppl.1), 187-196, 10 pp.
RFQ