No products in the cart.
Raltegravir EP Impurity E
Product Description
CAT No.
ALN-R009006
CAS No.
1193687-87-4
Mol. F.
C20H22N6O5
Mol. Wt.
426.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
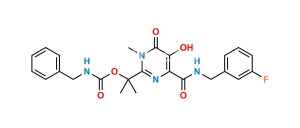
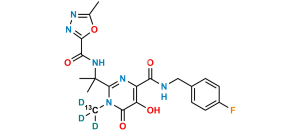
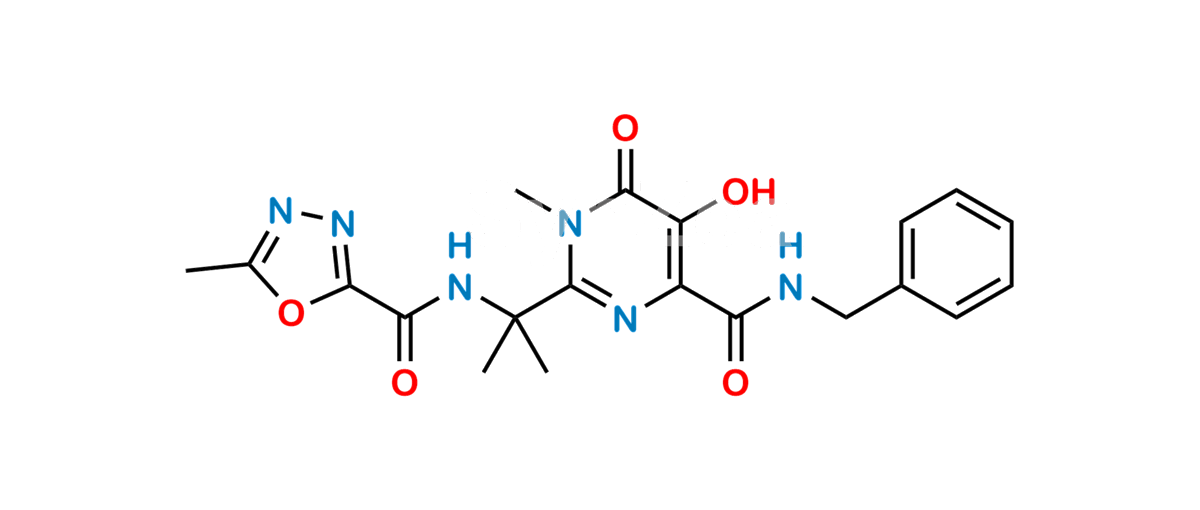
Chemical Name : N-benzyl-5-hydroxy-1-methyl-2-[2-(5-methyl-1,3,4-oxadiazole-2-carboxamido)propan-2-yl]-6-oxo-1,6-dihydropyrimidine-4-carboxamide (as per EP);
N-{2-[4-(Benzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl]propan-2-yl}-5-methyl-1,3,4-oxadiazole-2-carboxamide (as per USP)
Smiles : O=C(C(N=C(C(NC(C1=NN=C(C)O1)=O)(C)C)N2C)=C(O)C2=O)NCC3=CC=CC=C3
Inchi : InChI=1S/C20H23FN6O6/c1-10(28)25-26-17(32)16(31)24-20(2,3)19-23-13(14(29)18(33)27(19)4)15(30)22-9-11-5-7-12(21)8-6-11/h5-8,29H,9H2,1-4H3,(H,22,30)(H,24,31)(H,25,28)(H,26,32)
Synonym : Raltegravir USP Related Compound E
Technical Data
Reference
Development and validation of stability indicating RP-HPLC assay method of raltegravir in tablet dosage forms
By Shirisha, V.; Sairaju, B.; Illendula, Santhosh; Dutt, K. RajeswarnFrom World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(10), 668-692
Stability indicating reverse-phase high performance liquid chromatography method for the determination of raltegravir in bulk and pharmaceutical formulation
By Annapurna, Mukthinuthalapati Mathrusri; Teja, Gunnam Ravi; Hemchand, S.; Babu, R. Ravi Chandra – From International Journal of Green Pharmacy (2018), 12(1Suppl.), S177-S180
Development and validation of RP-HPLC method for determination of raltegravir and its impurities in bulk drug and dosage forms
By Balaji, M.; AppaRao, K. M. Ch.; Ramakrishna, K.; Srinivasarao, V. – From Pharma Science Monitor (2014), 5(3Suppl.1), 187-196, 10 pp.
RFQ