No products in the cart.
Ritonavir EP Impurity J

Product Description
CAT No.
ALN-R003011
CAS No.
162849-95-8
Mol. F.
C28H35N3O5S
Mol. Wt.
525.7
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
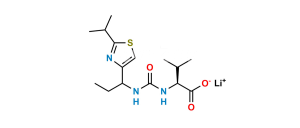
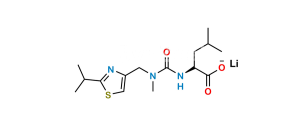
Chemical Name : Thiazol-5-yl-methyl [(1S,2S,4S)-1-benzyl4-[[(1,1-dimethylethoxy)carbonyl] amino]-2-hydroxy-5-phenylpentyl]carbamate (as per EP);
thiazol-5-ylmethyl (2S,3S,5S)-(5-t-butoxycarbonylamino)-3-hydroxy-1,6-diphenylhexan-2-ylcarbamate (as per USP)
Smiles : O[C@@H](C[C@@H](NC(OC(C)(C)C)=O)CC1=CC=CC=C1)[C@@H](NC(OCC2=CN=CS2)=O)CC3=CC=CC=C3
Inchi : InChI=1S/C36H46N6O5S2/c1-5-32-38-28(22-48-32)20-42(4)35(45)41-33(24(2)3)34(44)39-27(16-25-12-8-6-9-13-25)18-31(43)30(17-26-14-10-7-11-15-26)40-36(46)47-21-29-19-37-23-49-29/h6-15,19,22-24,27,30-31,33,43H,5,16-18,20-21H2,1-4H3,(H,39,44)(H,40,46)(H,41,45)/t27-,30-,31-,33-/m0/s1
Synonym : Ritonavir BOC-Aminoalcohol (USP)
Technical Data
Reference
Development and validation of stability-indicating UPLC-TUV method for simultaneous estimation of darunavir and ritonavir in bulk and tablet dosage form
By Kamalakannan, Dhanabalan; AnandaThangadurai, SubramaniamnFrom International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2021), 12(1), 611-619
Stability indicating method development and validation for simultaneous estimation of Lopinavir and Ritonavir by using RP-HPLC
By Raghu, P. S. – From World Journal of Pharmaceutical Research (2018), 7(3), 1750-1757
Stability-indicating RP-HPLC method for simultaneous quantification of ombitasvir, paritaprevir and ritonavir in tablet dosage form
By Kuna, Mangamma; Dannana, Gowri Sankar – From Asian Journal of Chemistry (2018), 30(6), 1277-1283
RFQ