No products in the cart.
Sacubitril Impurity 33

Product Description
CAT No.
ALN-S020041
CAS No.
219718-02-2
Mol. F.
C22H27NO4
Mol. Wt.
369.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
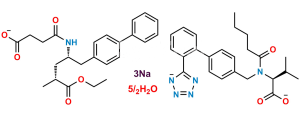
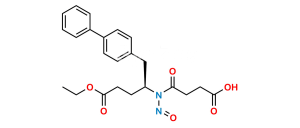
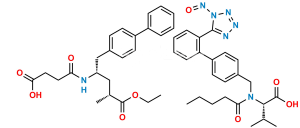
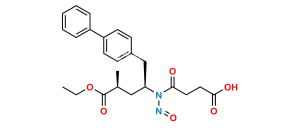
Chemical Name : (R)-3-([1,1′-Biphenyl]-4-yl)-2-((tert-butoxycarbonyl)amino)propyl acetate
Smiles : O=C(OC(C)(C)C)N[C@@H](COC(C)=O)CC1=CC=C(C2=CC=CC=C2)C=C1
Inchi : InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m0/s1
Technical Data
Reference
A liquid chromatographic method for separation of sacubitrilu2013valsartan and their stereoisomeric impurities
Lu Zhou,u00a0 Liang Zou,u00a0 Lili Sun,u00a0 Hui Zhang,u00a0 Wenkai Hui andu00a0 Qiaogen ZounAnal. Methods, 2018,10, 1046-1053
Quantification of potential genotoxic impurity in sacubitril/valsartan drug substance at ppm level by LC-MS/MS
Ravi Uppala, M.Arthanareeswari, S.Devikala,T.Pushpamalini, J. ArockiaSelvi – Materials Today: Proceedings Volume 14, Part 2, 2019, Pages 640-645
Experimental Design Approach in HPLC Method Development: Application for the Simultaneous Determination of Sacubitril and Valsartan in Presence of Their Impurities and Investigation of Degradation Kinetics
Bahia A. Moussa, Hanaa M. A. Hashem, Marianne A. Mahrouse & Sally T. Mahmoud – Chromatographia volume 81, pages139–156(2018)
RFQ