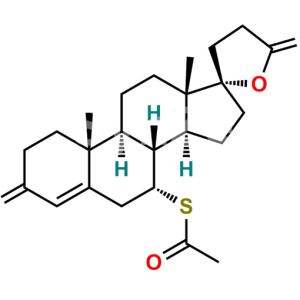
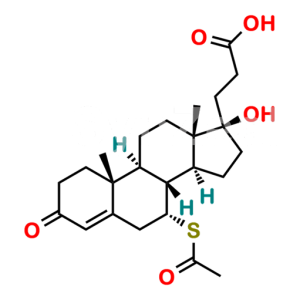
Spironolactone EP Impurity G

Product Description
CAT No.
ALN-S017008
CAS No.
880106-10-5
Mol. F.
C24H32O5S
Mol. Wt.
432.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
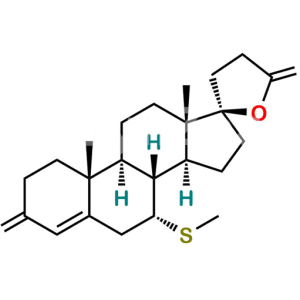
Chemical Name : S-[(2′R)-6β-Hydroxy-3,5′-dioxo-3′,4′-dihydro-5′H-spiro[androst-4-ene-17,2′-furan]-7α-yl] ethanethioate (as per EP)
Smiles : C[C@@]12[C@@]3(OC(CC3)=O)CC[C@@]1([H])[C@]4([H])[C@@](SC(C)=O)([H])[C@@H](O)C5=CC(CC[C@]5(C)[C@@]4([H])CC2)=O
Inchi : InChI=1S/C22H28O3/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21)12-8-19(24)25-22/h3-4,13,16-18H,5-12H2,1-2H3/t16-,17+,18+,20+,21+,22-/m1/s1
Synonym : 6β-Hydroxy Spironolactone
Technical Data
Reference
RP- HPLC method for the simultaneous estimation of losartan and spironolactone in tablet dosage form
By Chaure, Pravin P.; Sigh, Sonia; Shariff, Arshia; Wagh, Vishalkumar H.; Tandale, Sandeep D.nFrom Journal of Pharmaceutical Sciences and Research (2019), 11(8), 2866-2871.
Design of experimental approach to analytical robustness study for UHPLC method developed for separation and quantification of spironolactone and its impurities in drug substances
By Veerendra, Y. V. S.; Brahman, Pradeep Kumar; Mankumare, Sharad D.; Ch, Jaya Raju; Satish, J. – From Asian Journal of Chemistry (2020), 32(2), 219-227.
Influence of different chromatographic conditions on the level of detection and quantitation of spironolactone by means of TLC-densitometry
By Dolowy, Malgorzata – From Journal of Analytical Methods in Chemistry (2019), 8792783/1-8792783/8.
RFQ