No products in the cart.
Sunitinib N-Oxide

Product Description
CAT No.
ALN-S016012
CAS No.
356068-99-0
Mol. F.
C22H27FN4O3
Mol. Wt.
414.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
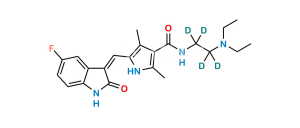
Chemical Name : N-[2-(Diethyloxidoamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide ;
Smiles : O=C(C1=C(C)NC(/C=C2C(NC3=C\2C=C(F)C=C3)=O)=C1C)NCC[N+](CC)(CC)[O-]
Inchi : InChI=1S/C25H31FN4O4/c1-7-30(24(33)34-25(4,5)6)11-10-27-23(32)21-14(2)20(28-15(21)3)13-18-17-12-16(26)8-9-19(17)29-22(18)31/h8-9,12-13,28H,7,10-11H2,1-6H3,(H,27,32)(H,29,31)/b18-13-
Technical Data
Reference
Physicochemical characteristics of sunitinib malate and its process-related impurities
Katarzyna Sidoryk 1, Maura Maliu0144ska, Krzysztof Bau0144kowski, Marek Kubiszewski, Marta u0141aszcz, Magdalena Bodziachowska-Panfil, Magdalena Kossykowska, Tomasz Giller, Andrzej Kutner, Krzysztof Wou017aniaknJ Pharm Sci. 2013 Feb;102(2):706-16.
Development and validation of a HPTLC method for analysis of Sunitinib malate
Monireh Hajmalek, Masoumeh Goudarzi , Solmaz Ghaffari,Hossein Attar, Mehrnoosh Ghanbari Mazlaghan – Brazilian Journal of Pharmaceutical Sciences vol. 52, n. 4, oct./dec., 2016
Reverse phase HPLC determination of sunitinib malate using UV detector, its isomerisation study, method development and validation
Mohsen Padervand, Solmaz Ghaffari, Hossein Attar & Mahdieh Mohammad Nejad – Journal of Analytical Chemistry volume 72, pages567–574(2017)
RFQ