No products in the cart.
Tamoxifen EP Impurity C Citrate

Product Description
CAT No.
ALN-T038020
CAS No.
20171-74-8
Mol. F.
C24H25NO : C6H8O7
Mol. Wt.
343.5 : 192.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
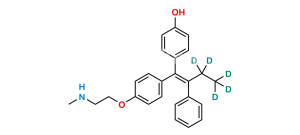
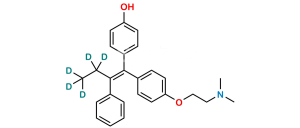
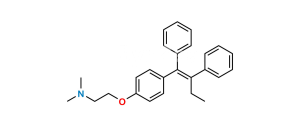
Chemical Name : 2-[4-[(EZ)-1,2-Diphenyleth-1-en-1-yl]phenoxy]-N,N-dimethylethan-1-amine 2-hydroxypropane-1,2,3-tricarboxylate
Smiles : CN(C)CCOC(C=C1)=CC=C1/C(C2=CC=CC=C2)=C/C3=CC=CC=C3.OC(CC(C(O)=O)(O)CC(O)=O)=O
Inchi : InChI=1S/C12H18N2O2/c1-14(2)8-5-9-16-12(15)10-6-3-4-7-11(10)13/h3-4,6-7H,5,8-9,13H2,1-2H3
Technical Data
Reference
Simultaneous Determination of Tamoxifen Citrate and Its E Isomer Impurity in Bulk Drug and Tablets by High-Performance Liquid Chromatography
Harry G.JalonennJournal of Pharmaceutical Sciences Volume 77, Issue 9, September 1988, Pages 810-813
Quantitative monitoring of tamoxifen in human plasma extended to 40 metabolites using liquid-chromatography high-resolution mass spectrometry: New investigation capabilities for clinical pharmacology
Elyes Dahmane, Julien Boccard, Chantal Csajka, Serge Rudaz – Analytical and Bioanalytical Chemistry 406(11)
Analysis of Tamoxifen and Its Metabolites by On-Line Capillary Electrophoresis?Electrospray Ionization Mass Spectrometry Employing Nonaqueous Media Containing Surfactants
Wenzhe Lu, Grace K. Poon, Paul L. Carmichael, and Richard B. Cole – Anal. Chem. 1996, 68, 4, 668–674
RFQ