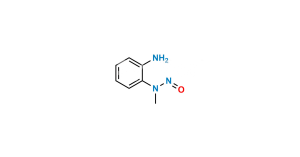
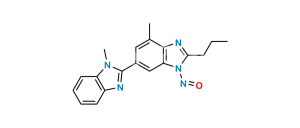
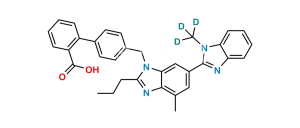
No products in the cart.
Telmisartan EP Impurity F
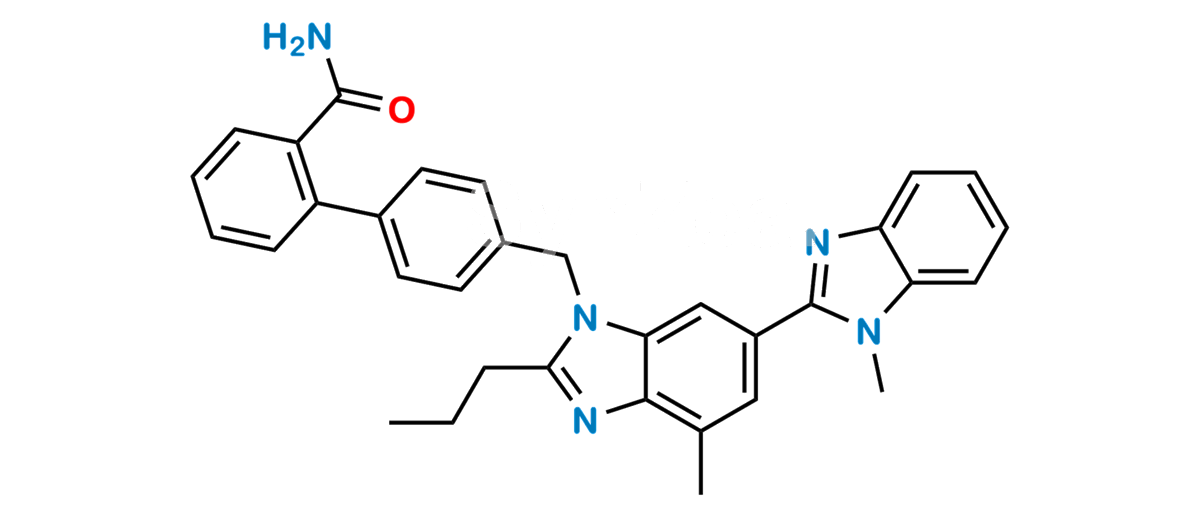
Product Description
CAT No.
ALN-T006006
CAS No.
915124-86-6
Mol. F.
C33H31N5O
Mol. Wt.
513.6
Stock
In Stock
Product Overview
Technical Data
Reference
RFQ
Product Overview
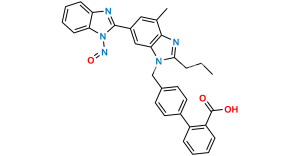
Chemical Name : 2-(4-{[4-Methyl-6-(1-methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl] methyl}phenyl)benzamide
Smiles : CC1=C2C(N(C(CCC)=N2)CC3=CC=C(C(C=CC=C4)=C4C(N)=O)C=C3)=CC(C5=NC6=CC=CC=C6N5C)=C1
Inchi : InChI=1S/C26H24N2O4/c1-3-6-23-27-24-16(2)13-19(25(29)30)14-22(24)28(23)15-17-9-11-18(12-10-17)20-7-4-5-8-21(20)26(31)32/h4-5,7-14H,3,6,15H2,1-2H3,(H,29,30)(H,31,32)
Synonym : Telmisartan Amide
Technical Data
Reference
Detection, isolation and characterization of principle synthetic route indicative impurity in telmisartan
V.Srinivasan, H.Sivaramakrishnan, B.KarthikeyannArabian Journal of Chemistry Volume 9, Supplement 2, November 2016, Pages S1516-S1522
Quality-by-design approach for the development of telmisartan potassium tablets
Ga-Hui Oh, Jin-Hyun Park, Hye-Won Shin, Joo-Eun Kim, Young-Joon Park – Drug Dev Ind Pharm. 2018 May;44(5):837-848.
An Efficient and Impurity-Free Process for Telmisartan:\u2009 An Antihypertensive Drug
Kikkuru Srirami Reddy, Neti Srinivasan, Chinta Raveendra Reddy, Naveenkumar Kolla, Yerremilli Anjaneyulu, Sundaram Venkatraman, Apurba Bhattacharya, and Vijayavitthal T. Mathad – Org. Process Res. Dev. 2007, 11, 1, 81–85
RFQ