No products in the cart.
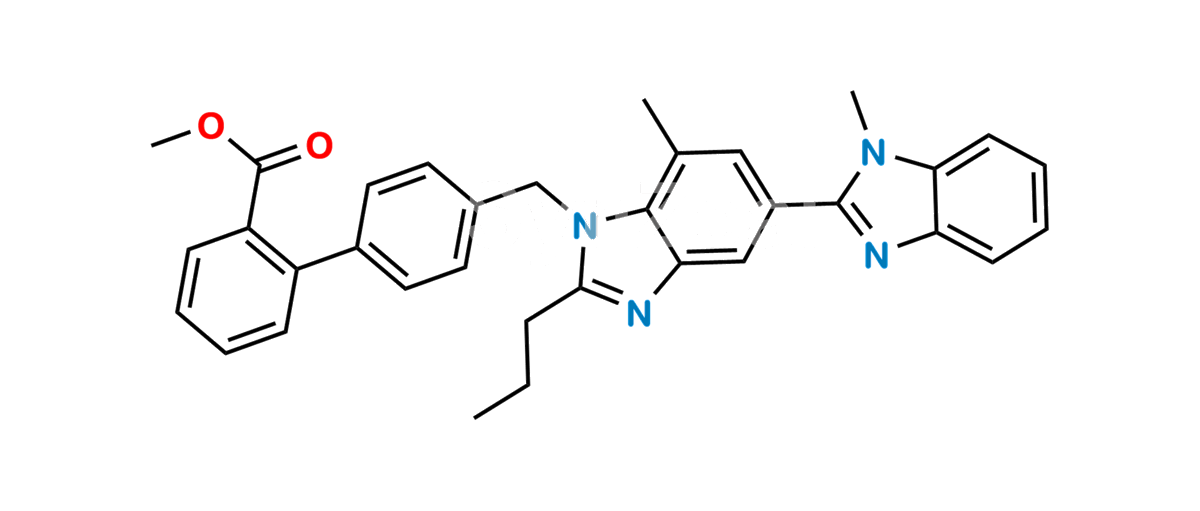
Telmisartan Impuroty B Methyl Ester
Product Description
CAT No.
ALN-T006022
CAS No.
1338830-37-7
Mol. F.
C34H32N4O2
Mol. Wt.
528.7
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
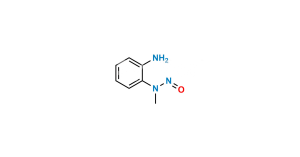
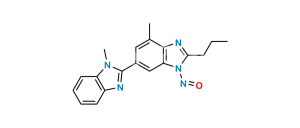
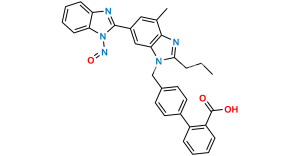
Chemical Name : Methyl 4′-((1,7′-dimethyl-2′-propyl-1H,1’H-[2,5′-bibenzo[d]imidazol]-1′-yl)methyl)-[1,1′-biphenyl]-2-carboxylate
Smiles : CC1=C2N(C(CCC)=NC2=CC(C3=NC4=CC=CC=C4N3C)=C1)CC5=CC=C(C(C=CC=C6)=C6C(OC)=O)C=C5
Inchi : InChI=1/C17H21NO/c1-14-8-6-7-11-16(14)18(2)13-12-17(19)15-9-4-3-5-10-15/h3-11,17,19H,12-13H2,1-2H3
Technical Data
Reference
Detection, isolation and characterization of principle synthetic route indicative impurity in telmisartan
V.Srinivasan, H.Sivaramakrishnan, B.KarthikeyannArabian Journal of Chemistry Volume 9, Supplement 2, November 2016, Pages S1516-S1522
Quality-by-design approach for the development of telmisartan potassium tablets
Ga-Hui Oh, Jin-Hyun Park, Hye-Won Shin, Joo-Eun Kim, Young-Joon Park – Drug Dev Ind Pharm. 2018 May;44(5):837-848.
An Efficient and Impurity-Free Process for Telmisartan:\u2009 An Antihypertensive Drug
Kikkuru Srirami Reddy, Neti Srinivasan, Chinta Raveendra Reddy, Naveenkumar Kolla, Yerremilli Anjaneyulu, Sundaram Venkatraman, Apurba Bhattacharya, and Vijayavitthal T. Mathad – Org. Process Res. Dev. 2007, 11, 1, 81–85
RFQ