No products in the cart.
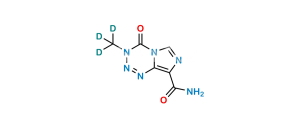
Temozolomide Impurity 2
Product Description
CAT No.
ALN-T002014
CAS No.
NA
Mol. F.
C16H26N4O11
Mol. Wt.
450.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : 5-(((2R,3R,4R,5S,6R)-3,4-Dihydroxy-6-(hydroxymethyl)-5-(((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)amino)-1H-imidazole-4-carboxamide
Smiles : O[C@@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](NC3=C(C(N)=O)N=CN3)O[C@@H]2CO)O[C@@H]1CO
Inchi : InChI=1/C12H17N.CH4O3S/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12;1-5(2,3)4/h3-6,12-13H,2,7-9H2,1H3;1H3,(H,2,3,4)
Synonym : 5-Aminoimidazole-4-Carboxamide Lactose Adduct
Technical Data
Reference
Design of experiment based validated stability indicating RP-HPLC method of temozolomide in bulk and pharmaceutical dosage forms
Anam Khan, Syed Sarim Imam, Mohd Aqil, Yasmin Sultana, Asgar Ali, Khalid KhannBeni-Suef University Journal of Basic and Applied Sciences Volume 5, Issue 4, December 2016, Pages 402-408
Identification and Physicochemical Characteristics of Temozolomide Process-Related Impurities
Marta ?aszcz, Marek Kubiszewski, ?ukasz Jedynak, Monika Kaczmarska, ?ukasz Kaczmarek, Wojciech ?uniewski, Krzysztof Gabarski, Anna Witkowska, Krzysztof Kuziak and Maura Mali?ska – Molecules 2013, 18, 15344-15356
Development and Validation of Simultaneous Detrmination of Anastrozole and Temozolomide in Pharmaceutical Dosage Forms
V.N. Daphal
RFQ