No products in the cart.
Tenofovir Impurity 35
Product Description
CAT No.
ALN-T012059
CAS No.
1962114-91-5
Mol. F.
C15H22N5O7P
Mol. Wt.
415.3
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
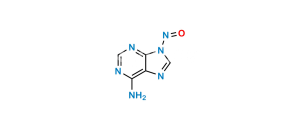
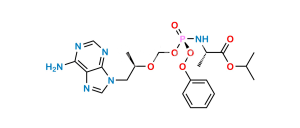
Chemical Name : ((hydroxy((((R)-1-(6-(methyleneamino)-9H-purin-9-yl)propan-2-yl)oxy)methyl)phosphoryl)oxy)methyl isopropyl carbonate
Smiles : OP(OCOC(OC(C)C)=O)(CO[C@H](C)CN1C=NC2=C(N=C)N=CN=C12)=O
Inchi : InChI=1S/C16H17NO/c1-12-7-8-16(18)15(11-12)14(9-10-17)13-5-3-2-4-6-13/h2-8,10-11,14,17-18H,9H2,1H3/t14-/m1/s1
Technical Data
Reference
Stability indicating RP-HPLC method development and validation for the simultaneous estimation of darunavir, cobicistat, emtricitabine and tenofovir alafenamide in bulk and pharmaceutical formulation
By Parameswari, S. Angala; Ankinapalli, Ajay Kumar Reddy; Tsegaye, Tesfaye; Alagusundaram, M.nFrom International Journal of Pharmaceutical Sciences and Research (2021), 12(6), 3216-3224
Stability-indicating high-performance thin-layer chromatography method for the simultaneous estimation of emtricitabine and tenofovir alafenamide fumarate
By Kashid, Arun M.; Kadam, Rohini R. – From Journal of Planar Chromatography–Modern TLC (2021), Ahead of Print
Method development and validation for multi-component analysis of lamivudine & tenofovir disoproxil fumarate in bulk drug by UV-visible spectrophotometer & RP-HPLC
By Sharma, Shweta; Ankalgi, Amar Deep; Kaushal, Pooja; Ashawat, M. S. – From Journal of Applied Pharmaceutical Research (2020), 8(4), 70-76
RFQ