No products in the cart.
Triamcinolone EP Impurity A
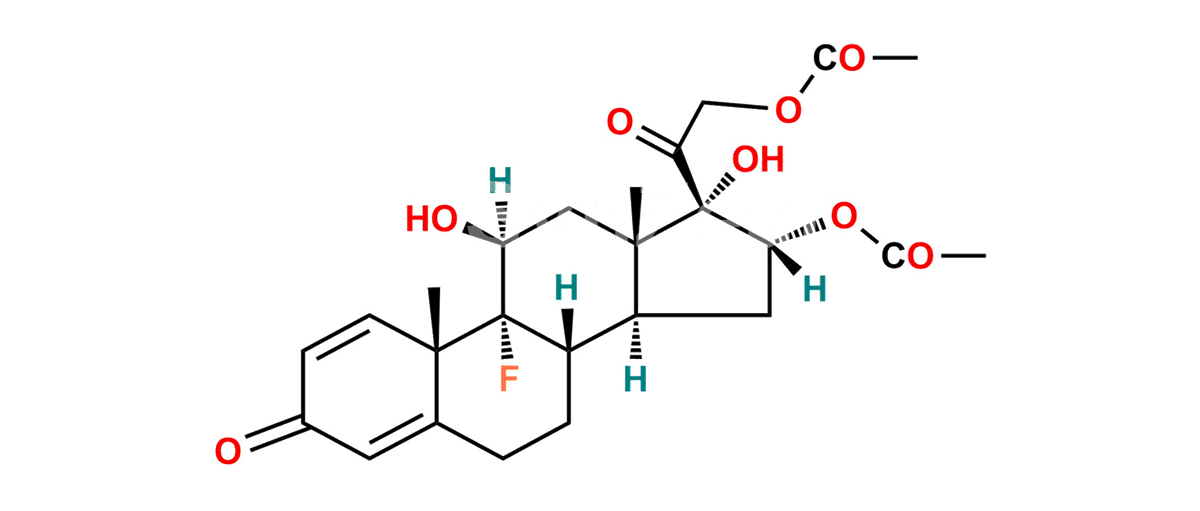
Product Description
CAT No.
ALN-T028002
CAS No.
67-78-7
Mol. F.
C25H31FO8
Mol. Wt.
478.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
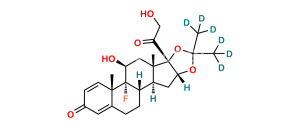
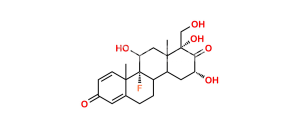
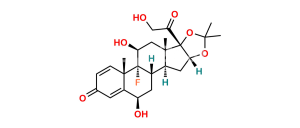
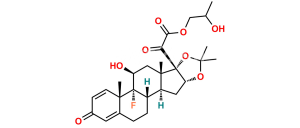
Chemical Name : 9-Fluoro-11β,17-dihydroxy-3,20-dioxopregna-1,4-diene-16α,21-diyl diacetate (as per EP);
9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 16,21-diacetate (as per USP)
Smiles : F[C@@]1([C@]2(C=C3)C)[C@](CCC2=CC3=O)([H])[C@@](C[C@](OC(C)=O)([H])[C@]4(O)C(COC(C)=O)=O)([H])[C@]4(C)C[C@]1([H])O
Inchi : InChI=1S/C21H27FO6/c1-18-6-5-12(24)7-11(18)3-4-13-14-8-15(25)21(28,17(27)10-23)19(14,2)9-16(26)20(13,18)22/h5-7,13-16,23,25-26,28H,3-4,8-10H2,1-2H3/t13-,14-,15+,16-,18-,19-,20-,21-/m0/s1
Synonym : Triamcinolone Diacetate ; Triamcinolone 16,21-Diacetate
Technical Data
Reference
u039414-steroidal impurity: preparation, characterization and evaluation, as well development of new HPLC method in drug substance like Triamcinolone Hexacetonide
By Shah, Tejas J.; Thakore, Anant R.; Chheda, Abhay H.; Desai, Geeta R.; Tandel, Harish S.nFrom Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry (2020), 8(1), 7-15
Resolution and quantitation of triamcinolone acetonide and its coformulated drug in the presence of its impurities and degradation products by HPTLC and HPLC
By Abbas, Samah S.; Hegazy, Maha A.; Hendawy, Hassan A. M.; Weshahy, Soheir A.; Abdelwahab, May H. – From Journal of AOAC International (2018), 101(4), 981-991
RFQ