No products in the cart.
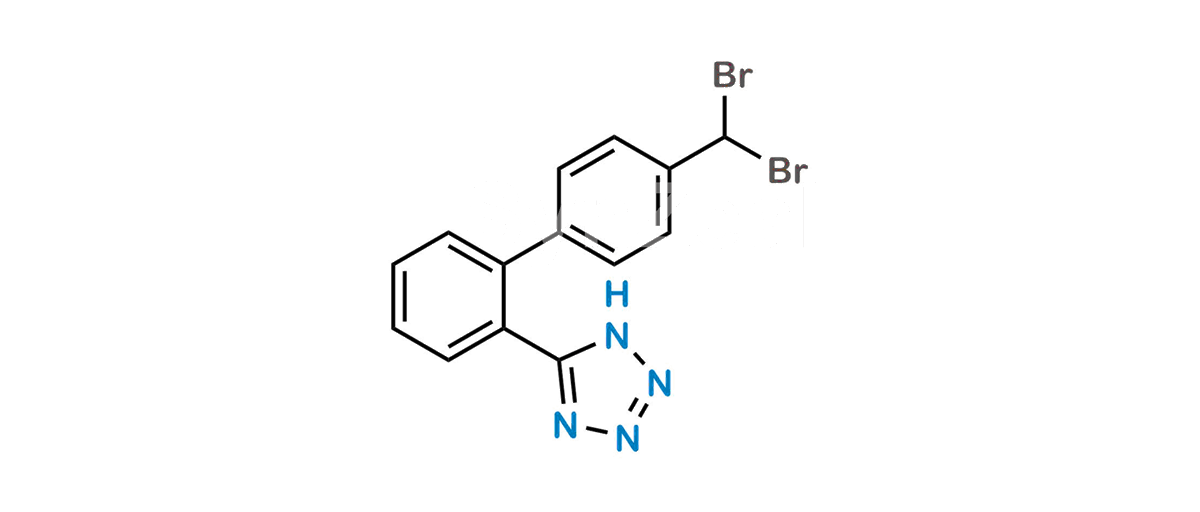
Valsartan Impurity 12
Product Description
CAT No.
ALN-V001033
CAS No.
1127249-17-5
Mol. F.
C14H10Br2N4
Mol. Wt.
394.1
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
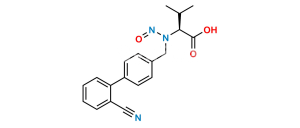
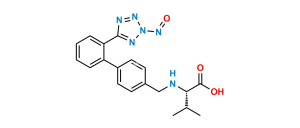
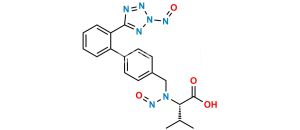
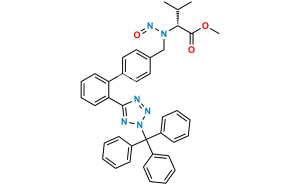
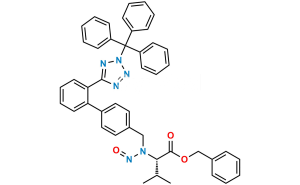
Chemical Name : 5-(4′-(Dibromomethyl)-[1,1′-biphenyl]-2-yl)-1H-tetrazole
Smiles : BrC(C1=CC=C(C2=CC=CC=C2C3=NN=NN3)C=C1)Br
Inchi : InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1
Technical Data
Reference
Identification and characterization of potential impurities of valsartan, AT1 receptor antagonist
A.Sampath, A. Raghupathi Reddy, B.Yakambaram, A.Thirupathi, M.Prabhakar, P. Pratap Reddy, V. Prabhakar ReddynJournal of Pharmaceutical and Biomedical Analysis Volume 50, Issue 3, 15 October 2009, Pages 405-412
Monitoring of Impurity Level of Valsartan and Hydrochlorothiazide Employing an RPu2013HPLC Gradient Mode
Darko Ivanovi?,An?elija Malenovi?,Biljana Jan?i?,Prof. Dr. Mirjana Medenica\xa0 &Marija Maškovi? – Journal of Liquid Chromatography & Related Technologies\xa0 Volume 30, 2007 – Issue 19 Pages 2879-2890
A liquid chromatographic method for separation of sacubitril–valsartan and their stereoisomeric impurities
Lu Zhou, Liang Zou, Lili Sun, Hui Zhang, Wenkai Hui and\xa0 Qiaogen Zou – Anal. Methods, 2018,10, 1046-1053
RFQ