No products in the cart.
Ziprasidone Impurity 8

Product Description
CAT No.
ALN-Z002021
CAS No.
2514708-83-7
Mol. F.
C11H14ClN3O2S
Mol. Wt.
287.8
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
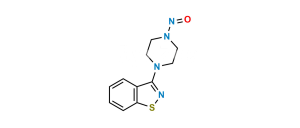
Chemical Name : 1-(1-oxidobenzo[d]isothiazol-3-yl)piperazine 1-oxide hydrochloride
Smiles : O=S1N=C([N+]2([O-])CCNCC2)C3=CC=CC=C31
Inchi : InChI=1S/C22H23ClN4OS/c1-25-19-14-18(23)15(12-16(19)13-21(25)28)6-7-26-8-10-27(11-9-26)22-17-4-2-3-5-20(17)29-24-22/h2-5,12,14H,6-11,13H2,1H3
Technical Data
Reference
Development and Validation of an HPLC Method for Determination of Ziprasidone and Its Impurities in Pharmaceutical Dosage Forms
Marija Pavlovic, Marija Malesevic, Katarina Nikolic, Danica AgbabanJournal of AOAC INTERNATIONAL, Volume 94, Issue 3, 1 May 2011, Pages 713u2013722
Optimization of the Thin-Layer Chromatography Method for the Separation of Ziprasidone and Its Impurities
Darija Obradovi?, Slavica Filipic, Katarina Nikolic & Danica Agbaba – JPC – Journal of Planar Chromatography – Modern TLC volume 29, pages239–246(2016)
Optimization of TLC method for separation and determination of ziprasidone and its impurities
\xa0Darija Obradovi?,Slavica Filipi?,Katarina Nikoli?,Marija ?arapi? &Danica Agbaba – Journal of Liquid Chromatography & Related Technologies Volume 39, 2016 – Issue 5-6: Special Issue: Thin Layer Chromatography Pages 271-276
RFQ