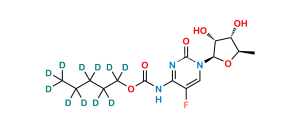
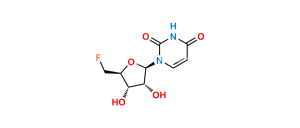
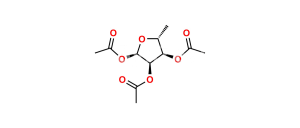
Capecitabine Impurity 4

Product Description
CAT No.
ALN-C003019
CAS No.
1313238-38-8
Mol. F.
C25H36FN3O10
Mol. Wt.
557.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
Chemical Name : Cytidine, 5′-deoxy-5-fluoro-N,N-bis[(pentyloxy)carbonyl]-, 2′,3′-diacetate
Smiles : O=C1N=C(N(C(OCCCCC)=O)C(OCCCCC)=O)C(F)=CN1[C@H]2[C@@H]([C@@H]([C@@H](C)O2)OC(C)=O)OC(C)=O
Inchi : InChI=1S/C15H23N3O6/c1-3-4-5-8-23-15(22)17-10-6-7-18(14(21)16-10)13-12(20)11(19)9(2)24-13/h6-7,9,11-13,19-20H,3-5,8H2,1-2H3,(H,16,17,21,22)/t9-,11-,12-,13-/m1/s1
Technical Data
Reference
Design of experiments (DoE) - based enhanced quality by design approach to hydrolytic degradation kinetic study of capecitabine by eco-friendly stability indicating UV-visible spectrophotometry
By Prajapati, Pintu; Patel, Radhika; Patel, Dilan; Shah, ShaileshnFrom American Journal of PharmTech Research (2020), 10(6), 115-133
Validated stability indicating HPLC method for estimation of capecitabine
By Lalitha, K. V.; Raveendra Reddy, J.; Devanna, N. – From International Journal of Pharmaceutical Sciences and Research (2020), 11(11), 5651-5658
RP-HPLC method development and validation for the simultaneous estimation of Irinotecan hydrochloride and Capecitabine in Active Pharmaceutical Ingredients (APIs)
By Vijaya Jyothi, M.; Bhargav, E.; Keerthana, B.; Varalakshmi, Devi K. – From International Journal of Research in Pharmaceutical Sciences (Madurai, India) (2018), 9(1), 63-67
RFQ