No products in the cart.
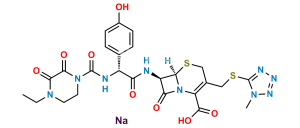
Cefoperazone Impurity 6

Product Description
CAT No.
ALN-C077013
CAS No.
NA
Mol. F.
C23H25N5O8S
Mol. Wt.
531.6
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
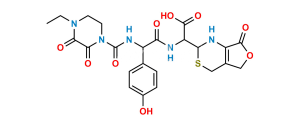
Chemical Name : (6R,7R)-7-((S)-2-(4-Ethyl-2,3-dioxopiperazine-1-carboxamido)-2-(4-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Smiles : O=C(N[C@H]1[C@]2([H])N(C(C(O)=O)=C(C)CS2)C1=O)[C@@H](NC(N3CCN(CC)C(C3=O)=O)=O)C4=CC=C(O)C=C4
Inchi : InChI=1S/C24H25N5O8S2/c1-2-27-7-8-28(21(34)20(27)33)24(36)26-15(12-3-5-13(30)6-4-12)18(31)25-16-19(32)29-17-14(11-39-22(16)29)38-10-9-37-23(17)35/h3-6,15-16,22,30H,2,7-11H2,1H3,(H,25,31)(H,26,36)/t15-,16-,22-/m1/s1
Technical Data
Reference
Development and Validation of Stability Indicating HPLC Method for the Determination of Impurities in the Sterile Mixture of Cefoperazone and Sulbactam
By Ivaturi, Ramu; Sastry, Thuttagunta Manikya; Sunkara, SatyaveninFrom Current Pharmaceutical Analysis (2019), 15(7), 762-775
Stability indicating method development and validation for simultaneous estimation of cefoperazone and tazobactam by using RP-HPLC
By Vytla, Sri Viswa Madhuri; Prasad, S. V. U. M.; Kuna, Mangamma – From Indo American Journal of Pharmaceutical Sciences (2017), 4(7), 2195-2203
Development and validation of HPLC and HPTLC methods for determination of cefoperazone and its related impurities
By Abdelaleem, Eglal A.; Naguib, Ibrahim A.; Zaazaa, Hala E.; Hussein, Essraa A. – From Journal of Chromatographic Science (2016), 54(2), 179-186
RFQ