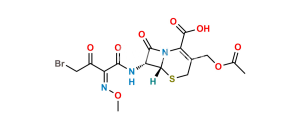
Cefotaxime Sodium EP Impurity F

Product Description
CAT No.
ALN-C126007
CAS No.
175032-97-0
Mol. F.
C30H29N9O12S5
Mol. Wt.
867.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
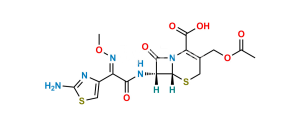
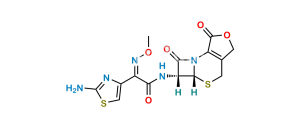
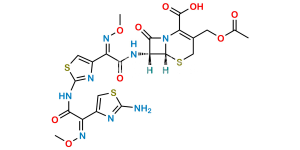
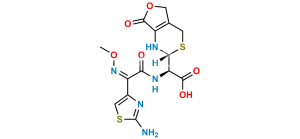
Chemical Name : (6R,7R)-3-[(Acetyloxy)methyl]-7-[[(2Z)-2-[2-[[[(6R,7R)-7-[[(2Z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1azabicyclo[4.2.0]oct-2-en-2-yl]methyl]amino]thiazol-4-yl]-2-(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (cefotaxime dimer)
Smiles : O=C1N2[C@@](SCC(COC(C)=O)=C2C(O)=O)([H])[C@]1([H])NC(/C(C3=CSC(SCC4=C(C(O)=O)N(C([C@]5(NC(/C(C6=CSC(N)=N6)=N\OC)=O)[H])=O)[C@]5([H])SC4)=N3)=N\OC)=O
Technical Data
Reference
Development and validation of stability indicating UFLC method for the estimation of Cefotaxime sodium and Diclofenac sodium in bulk and pharmaceutical dosage forms
By Koganti, Venkata Sairam; Thejaswini, J. Channabasappa; Chandan, Ravandur Shivanna; Bannimath, GurupadayyanFrom International Journal of Pharmaceutical Sciences Review and Research (2014), 25(1), 310-314, 5 pp..
Physical and chemical stability studies on cefotaxime and its dosage forms by stability indicating HPTLC method
By Behin, S.; Punitha, I. S. R.; Krishnan, S. – From International Journal of Pharmaceutical, Chemical and Biological Sciences (2012), 2(4), 517-523
Identification and characterization of new impurity in cefotaxime sodium drug substance
By Kumar, Vundavilli Jagadeesh; Gupta, Peruri Badarinadh; Pavan Kumar, K. S. R.; Prasada Rao, Korrapati V. V.; Prasanna, S. John; Siva Kumar, G. S.; Sharma, Hemant; Mukkanti, K. – From Pharma Chemica (2010), 2(3), 230-241
RFQ