No products in the cart.
Clarithromycin Impurity 3
Product Description
CAT No.
ALN-C040023
CAS No.
NA
Mol. F.
C38H67NO14
Mol. Wt.
761.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
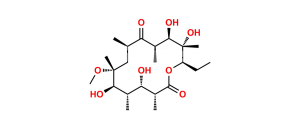
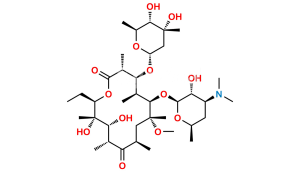
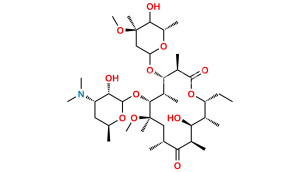
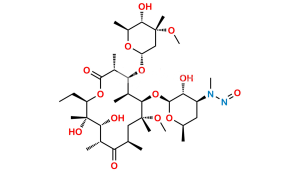
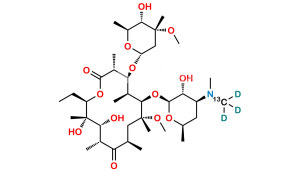
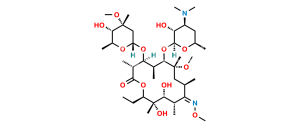
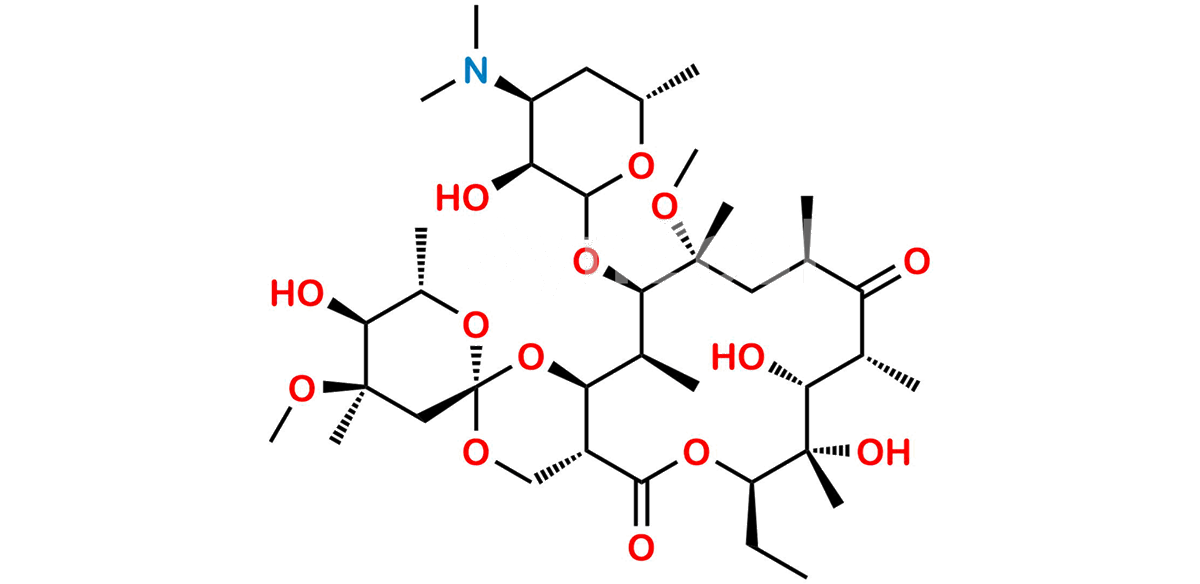
Chemical Name : (2S,4R,4a’R,5S,6S,7’R,8’S,9’R,10’R,12’R,14’R,15’R,16’S,16a’S)-15′-(((3S,4S,6S)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-7′-ethyl-5,8′,9′-trihydroxy-4,14′-dimethoxy-4,6,8′,10′,12′,14′,16′-heptamethylhexadecahydro-5’H,11’H-spiro[pyran-2,2′-[1,3]dioxino[5,4-c][1]oxacyclotetradecine]-5′,11′-dione
Smiles : C[C@H]([C@@H](O)[C@@](O)(C)[C@@H](CC)O1)C([C@H](C)C[C@](OC)(C)[C@H](OC2[C@@H](O)[C@@H](N(C)C)C[C@H](C)O2)[C@@H](C)[C@H](O[C@]3(C[C@](OC)(C)[C@@H](O)[C@H](C)O3)OC4)[C@@H]4C1=O)=O
Technical Data
Reference
Stability indicating HPLC-ECD method for the analysis of clarithromycin in pharmaceutical dosage forms: method scaling versus re-validation
By Makoni, Pedzisai A.; Chikukwa, Mellisa T. R.; Khamanga, Sandile M. M.; Walker, Roderick B.nFrom Scientia Pharmaceutica (2019), 87(4), 31
Stability indicating Rp-Hplc method development and validation for the simultaneous estimation of gemcitabine and clarithromycin in bulk and pharmaceutical dosage form
By Kumar, Y. Anil; Sirisha, Y.; Harini, A. L.; Prathyusha, A. – From World Journal of Pharmaceutical Research (2018), 7(8Spec.Iss.), 1-8
RFQ