No products in the cart.
Rifaximin Impurity 2
Product Description
CAT No.
ALN-R027011
CAS No.
1351775-03-5
Mol. F.
C43H49N3O11
Mol. Wt.
783.9
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
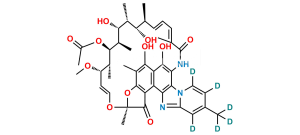
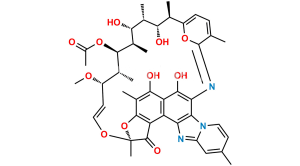
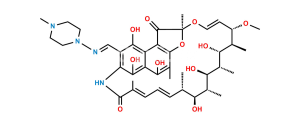
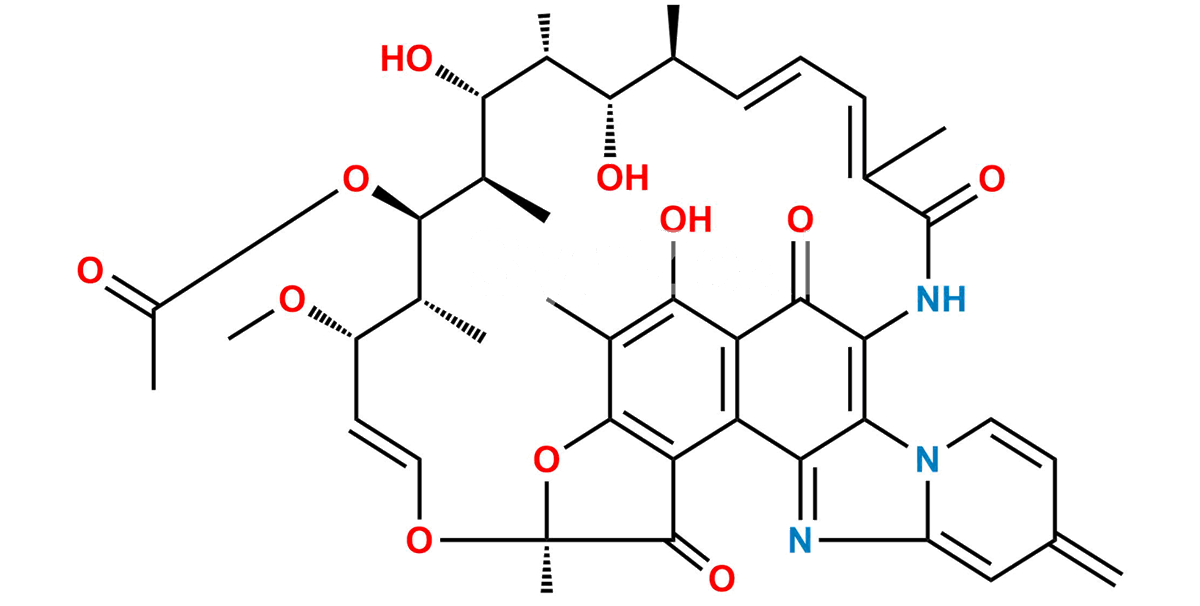
Smiles : O=C1C(C(C(C(N2C=C3)=C4NC(/C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@H]5C)=O)=NC2=CC3=C)=C6C4=O)=C(C(C)=C6O)O[C@@]1(O/C=C/[C@@H]([C@H]([C@H]5OC(C)=O)C)OC)C
Inchi : InChI=1S/C43H49N3O11/c1-19-14-16-46-28(18-19)44-32-29-30-37(50)25(7)40-31(29)41(52)43(9,57-40)55-17-15-27(54-10)22(4)39(56-26(8)47)24(6)36(49)23(5)35(48)20(2)12-11-13-21(3)42(53)45-33(34(32)46)38(30)51/h11-18,20,22-24,27,35-36,39,48-49,51H,1H2,2-10H3,(H,45,53)/b12-11-,17-15-,21-13-/t20-,22+,23+,24+,27-,35-,36+,39+,43-/m0/s1
Technical Data
Reference
Structural elucidation of the Rifaximin Ph. Eur. Impurity H
Riccardo Stradi, Donatella Nava, Marino Nebuloni, Barbara Pastura, ElenaPininJournal of Pharmaceutical and Biomedical Analysis Volume 51, Issue 4, 11 March 2010, Pages 858-865
Structure Elucidation of Two Unknown Oxydic Degradation Impurities of Rifaximin
chao liu, chang-qin hu\xa0 and shao-hong jin – Asian Journal of Chemistry; Vol. 23, No. 7 (2011), 3252-3256
Development and validation of rp-hplc method for the estimation of rifaximin in bulk and in tablet dosage form
T.sudha, P.V.hemalatha, V.R.ravikumar,r.jothi, M.radhakrishnan – Asian Journal of Pharmaceutical and Clinical Research Vol.2 Issue 4, October-December 2009
RFQ