Ceftriaxone EP Impurity B

Product Description
CAT No.
ALN-C082003
CAS No.
66340-33-8
Mol. F.
C14H13N5O5S2
Mol. Wt.
395.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
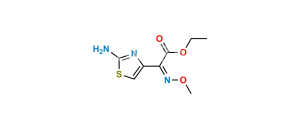
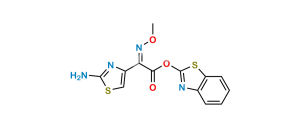
Chemical Name : (5aR,6R)-6-[[(2Z)-(2-Aminothiazol-4-yl)(methoxyimino)acetyl]amino]-5a,6-dihydro-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazine-1,7(4H)-dione (as per EP) ; (Z)-2-(2-Aminothiazol-4-yl)-N-{(5aR,6R)-1,7-dioxo-1,3,4,5a,6,7-hexahydroazeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl}-2-(methoxyimino)acetamide (as per USP)
Smiles : [H][C@@]12[C@@H](C(N1C3=C(CS2)COC3=O)=O)NC(/C(C4=CSC(N)=N4)=N\OC)=O
Inchi : InChI=1S/C18H18N8O7S3/c1-25-18(22-12(28)13(29)23-25)36-4-6-3-34-15-9(14(30)26(15)10(6)16(31)32)21-11(27)8(24-33-2)7-5-35-17(19)20-7/h5,9,15H,3-4H2,1-2H3,(H2,19,20)(H,21,27)(H,23,29)(H,31,32)/b24-8+/t9-,15-/m1/s1
Synonym : Deacetylcefotaxime lactone ; Cefotaxime EP Impurity E ; Cefotaxime USP Related Compound E
Technical Data
Reference
Stability Indicating Chromatographic Methods For Simultaneous Determination of Ceftriaxone Sodium and Sulbactam Sodium in their Combined Dosage Form
By EL-Bagary, Ramzia I.; Abo-talib, Nisreen F.; El-Hakeem, Maha M.; N. Eldin, M. BadawinFrom Current Pharmaceutical Analysis (2018), 14(5), 461-474
Stability-indicating spectrophotometric methods for determination of ceftriaxone in presence of its alkaline degradation product
By Attia, Khalid A. M.; Nassar, Mohammed W. I.; Abdul, Aziz M. M. El-Attar – From Analytical Chemistry: An Indian Journal (2016), 16(9), 398-405
RFQ