No products in the cart.
Celecoxib Nitroso Impurity 1

Product Description
CAT No.
ALN-C005017
CAS No.
NA
Mol. F.
C6H7N3O3S
Mol. Wt.
201.2
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
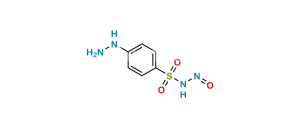
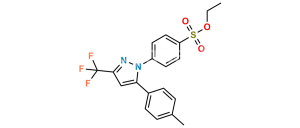
Chemical Name : 4-Amino-N-nitrosobenzenesulfonamide
Smiles : NC1=CC=C(S(NN=O)(=O)=O)C=C1
Inchi : InChI=1S/C7H12N2OS/c1-5-4-11-7(2-8)9-6(5)3-10/h3,7,9H,2,4,8H2,1H3/t7-/m1/s1
Technical Data
Reference
Development of a Unified Reversed-Phase HPLC Method for Efficient Determination of EP and USP Process-Related Impurities in Celecoxib Using Analytical Quality by Design Principles
Tim Tome, Zdenko u010casar ,OrcID andAleu0161 ObrezanMolecules 2020, 25(4), 809
A selective and sensitive LC-MS/MS method for the simultaneous determination of twopotential genotoxic impurities in celecoxib
Ambavaram Vijaya Bhaskar Reddy, Nandigam Venugopal & Gajulapalle Madhavi – Journal of Analytical Science and Technology volume 5, Article number: 18 (2014)
Stability-Indicating HPLC Method for Quantification of Celecoxib and Diacerein Along With Its Impurities in Capsule Dosage Form
Hanimi Reddy Bapatu, Ravi Kumar Maram, R. Satyanarayana Murthy – Journal of Chromatographic Science, Volume 53, Issue 1, January 2015, Pages 144–153
RFQ