No products in the cart.
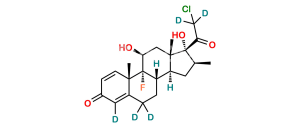
Clobetasol Propionate EP Impurity J

Product Description
CAT No.
ALN-C050011
CAS No.
1486466-31-2
Mol. F.
C25H30ClFO4
Mol. Wt.
449
Stock
In Stock
Product Overview
Technical Data
Reference
RFQ
Product Overview
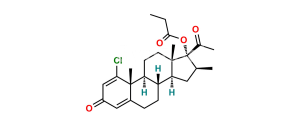
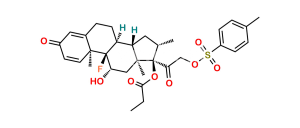
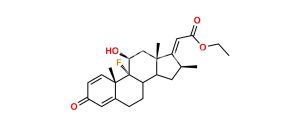
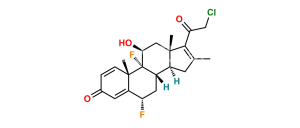
Chemical Name : (17R)-4′-Chloro-5′-ethyl-9-fluoro-11β-hydroxy-16β-methylspiro[androsta-1,4-diene-17,2′(3′H)-furan]-3,3′-dione ( 17α-spiro compound) (as per EP); 9α-Fluoro-11β-hydroxy-16β-methyl 3-oxo-androsta-1,4-diene-17(R)-spiro-2′-[4′-chloro-5′-ethylfuran-3′(2′H)-one] (as per USP)
Smiles : [H][C@@]12C[C@@H]([C@@]3([C@]1(C[C@@H]([C@]4([C@]2(CCC5=CC(C=C[C@]45C)=O)[H])F)O)C)C(C(Cl)=C(CC)O3)=O)C
Inchi : InChI=1S/C24H36O4S/c1-5-28-14-24(27)11-8-19-21-18(7-10-23(19,24)4)22(3)9-6-17(26)12-16(22)13-20(21)29-15(2)25/h12,18-21,27H,5-11,13-14H2,1-4H3/t18-,19-,20+,21+,22-,23-,24+/m0/s1
Synonym : Clobetasol Propionate USP Related Compound A
Technical Data
Reference
Development and validation of a fast RP-HPLC method for the determination of clobetasol propionate in topical nanocapsule suspensions
By Fontana M C; Bastos M O; Beck R C RnFrom Journal of chromatographic science (2010), 48(8), 637-40
Qualitative and quantitative assessment of related substances in the Compound Ketoconazole and Clobetasol Propionate Cream by HPLC-TOF-MS and HPLC
By Yang Wenling; Yang Xiaomei; Shi Fanghua; Wang Ruixun; Li Qing; Bi Kaishun; Liao Zhigang; Liang Yongkun; Yu Liangzhong – From Journal of pharmaceutical analysis (2019), 9(3), 156-162
RFQ