No products in the cart.
Rivaroxaban EP Impurity I

Product Description
CAT No.
ALN-R017053
CAS No.
1151893-81-0
Mol. F.
C24H21Cl2N3O7S2
Mol. Wt.
598.5
Stock
In Stock
Product Overview
Technical Data
Reference
RFQ
Product Overview
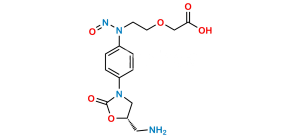
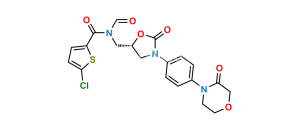
Chemical Name : [2-[15,95-dichloro-2,52,8-trioxo-3,7-diaza-5(3,5)-1,3-oxazolidina-1,9(2)-dithiophena-4(1,4)-benzenanonaphan-3-yl]ethoxy]acetic acid (as per EP)
Smiles : O=C(O)COCCN(C(C1=CC=C(Cl)S1)=O)C2=CC=C(N3C(O[C@@H](CNC(C4=CC=C(Cl)S4)=O)C3)=O)C=C2
Inchi : InChI=1S/C5H11NO2.ClH/c1-3-4(6)5(7)8-2;/h4H,3,6H2,1-2H3;1H/t4-;/m0./s1
Synonym : Rivaroxaban N-acyl glycolic acid (USP)
Technical Data
Reference
Development and validation of stability indicating UPLC method for the determination of Rivaroxaban in bulk and finished products and identification of degradation products by LCMS
By Ali, Syed Mastan; Ramachandran, D.nFrom Journal of Applicable Chemistry (Lumami, India) (2021), 10(3), 291-301
Validated LC-MS/MS method for the determination of three genotoxic impurities in rivaroxaban drug substance
By Korlakunta, Vijaya Gouri; Dasari, Vijaya Bharathi; Sharma, Hemant Kumar; Nowduri, Annapurna; Bonige, Kishore Babu; Chavakula, Ramadas – From European Journal of Biomedical and Pharmaceutical Sciences (2020), 7(8), 492-500
Development of a stability-indicating HPLC method and a dissolution test for rivaroxaban dosage forms
By Souri, Effat; Mottaghi, Siavash; Zargarpoor, Mohammad; Ahmadkhaniha, Reza; Jalalizadeh, Hassan – From Acta Chromatographica (2016), 28(3), 347-361
RFQ