No products in the cart.
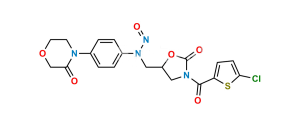
Rivaroxaban EP Impurity H

Product Description
CAT No.
ALN-R017054
CAS No.
1770812-37-7
Mol. F.
C19H17Cl2N3O5S
Mol. Wt.
470.3
Stock
In Stock
Product Overview
Technical Data
Reference
RFQ
Product Overview
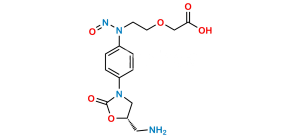
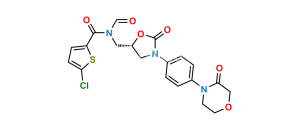
Chemical Name : 4,5-dichloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide (as per EP);(S)-4,5-Dichloro-N-({2-oxo-3-[4-(3-oxomorpholino)phenyl]oxazolidin-5-yl}methyl)thiophene-2-carboxamide (as per USP)
Smiles : O=C(C1=CC(Cl)=C(Cl)S1)NC[C@H]2CN(C3=CC=C(N4C(COCC4)=O)C=C3)C(O2)=O
Inchi : InChI=1S/C24H21Cl2N3O7S2/c25-19-7-5-17(37-19)22(32)27-11-16-12-29(24(34)36-16)15-3-1-14(2-4-15)28(9-10-35-13-21(30)31)23(33)18-6-8-20(26)38-18/h1-8,16H,9-13H2,(H,27,32)(H,30,31)/t16-/m0/s1
Synonym : Rivaroxaban USP Related Compound H
Technical Data
Reference
Development and validation of stability indicating UPLC method for the determination of Rivaroxaban in bulk and finished products and identification of degradation products by LCMS
By Ali, Syed Mastan; Ramachandran, D.nFrom Journal of Applicable Chemistry (Lumami, India) (2021), 10(3), 291-301
Validated LC-MS/MS method for the determination of three genotoxic impurities in rivaroxaban drug substance
By Korlakunta, Vijaya Gouri; Dasari, Vijaya Bharathi; Sharma, Hemant Kumar; Nowduri, Annapurna; Bonige, Kishore Babu; Chavakula, Ramadas – From European Journal of Biomedical and Pharmaceutical Sciences (2020), 7(8), 492-500
Development of a stability-indicating HPLC method and a dissolution test for rivaroxaban dosage forms
By Souri, Effat; Mottaghi, Siavash; Zargarpoor, Mohammad; Ahmadkhaniha, Reza; Jalalizadeh, Hassan – From Acta Chromatographica (2016), 28(3), 347-361
RFQ