No products in the cart.
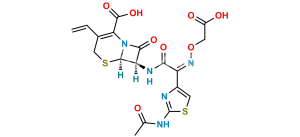
Cefixime Impurity 3

Product Description
CAT No.
ALN-C081017
CAS No.
128012-77-1
Mol. F.
C16H15N5O7S2
Mol. Wt.
453.4
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
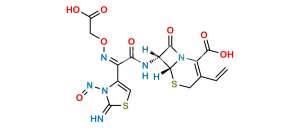
Chemical Name : 2-((((Z)-1-(2-Aminothiazol-4-yl)-2-(((6aR,7R)-1,8-dioxo-1,3,4,5,6a,7-hexahydro-8H-azeto[2,1-b]pyrano[3,4-d][1,3]thiazin-7-yl)amino)-2-oxoethylidene)amino)oxy)acetic acid
Smiles : O=C1N2C(C3=O)=C(CCO3)CS[C@]2([H])[C@@H]1NC(/C(C4=CSC(N)=N4)=N\OCC(O)=O)=O
Inchi : InChI=1/C16H15N5O7S2/c1-5-6-3-29-14-10(13(25)21(14)11(6)15(26)28-5)19-12(24)9(20-27-2-8(22)23)7-4-30-16(17)18-7/h4-5,10,14H,2-3H2,1H3,(H2,17,18)(H,19,24)(H,22,23)/b20-9+
Technical Data
Reference
Analytical method development and validation of cefixime and azithromycin by using RP-HPLC
By Sireesha, E.; Reddy, L. Ramachandra; Dhachinamoorthi, D.nFrom World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(9), 1160-1177
Stability indicating hplc method for the quantification of cefixime, ornidazole and moxifloxacin in solid dosage forms
By Palacharla, Suresh Kumar; Mohan, G. V. Krishna – From Rasayan Journal of Chemistry (2018), 11(4), 1696-1714
Validated stability-indicating HPTLC method for cefixime and azithromycin with preparative isolation, identification, and characterization of degradation products
By Gawande, V. T.; Bothara, K. G.; Satija, C. O. – From Acta Chromatographica (2018), 30(4), 212-218
RFQ