No products in the cart.
Ticagrelor Impurity 112

Product Description
CAT No.
ALN-T023200
CAS No.
220352-32-9
Mol. F.
C20H23F2NO3S
Mol. Wt.
395.5
Stock
Please Inquire
Product Overview
Technical Data
Reference
RFQ
Product Overview
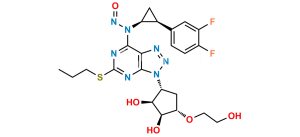
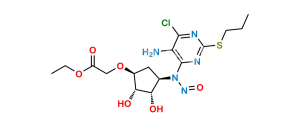
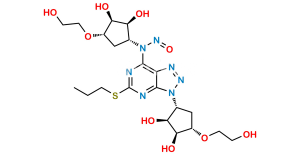
Chemical Name : ((1R,2R)-2-(3,4-Difluorophenyl)cyclopropyl)((6R,7aR)-8,8-dimethyl-2,2-dioxidotetrahydro-3H-3a,6-methanobenzo[c]isothiazol-1(4H)-yl)methanone
Smiles : CC1(C2(C[S]3(=O)=O)[C@@](N3C([C@H]4[C@H](C5=CC(F)=C(F)C=C5)C4)=O)([H])C[C@@]1([H])CC2)C
Inchi : InChI=1S/C19H21F2NO3S/c1-18(2)13-7-8-19(18)11-26(24,25)22(16(19)10-13)17(23)6-4-12-3-5-14(20)15(21)9-12/h3-6,9,13,16H,7-8,10-11H2,1-2H3/b6-4+/t13-,16+,19?/m0/s1
Technical Data
Reference
Estimation Of Ticagrelor In Commercial Dosage Form Using A Sensitive Validated RP-HPLC Method
By Madhuri, K.; Rao, Y. Srinivasa; Varaprasada, Rao K.; Deepthi, R.nFrom World Journal of Pharmaceutical Research (2021), 10(6), 1281-1292.
Development and validation of stability indicating RP-HPLC method for the estimation of Ticagrelor by forced degradation studies
By Dole, Manjusha; Kendre, Meenakshi; Wagh, Viplav – From World Journal of Pharmacy and Pharmaceutical Sciences (2019), 8(5), 711-724
Development of a validated HPLC-PDA method for stability indicating study of ticagrelor: a novel anti-platelet agent (P2Y12-ADP receptor blocker)
By Sulthana, Shabana; Anuradha, V.; Rao, Mandava V. Basaveswara – From International Journal of Pharmaceutical, Chemical and Biological Sciences (2017), 7(1), 36-42
RFQ